

DEPRIESTER CHART ONLINE FREE
HW #4, P11.36 - Excess Gibbs Free Energy of a Real.Phase Equilibrium (Alternate Form VLE) Historically, when estimates were done by hand:.HW #4, P11.28 - Excess Gibbs Free Energy of a Real.In order to maximize the advantages of your EHR, you will desire to pre-populate the electronic chart with important clinical and market details. Typically it is helpful to develop charts that compare different varieties of data. HW #4, P10.31+ - Equilibrium Flash Distillation U. Chart are incredibly helpful method to picture information.
DEPRIESTER CHART ONLINE HOW TO
HW #4, P10.25+ - Bubble and Dew Point Calculation. This Demonstration applies a DePriester chart a set of nomograms to find the vaporliquid equilibrium ratio the gas phase mole fraction divided by the liquid. Describes how to use an interactive simulation that demonstrates how to read vapor-liquid equilibrium ratios from a DePriester chart.HW #4, P11.25 - Fugacity of a Mixture: Real vs.HW #5, P12.27 - Volume Change of Mixing Two Liquid.HW #5, P12.22 - Multicomponent Flash Using the Wil.HW #5, WB.4 - Bubble Point and Dew Point Calculati.HW #5, WB.3 - Determination of Azeotropes Using th.HW #5, WB.2 - Determination of Azeotropes Using M.Bishop NJ, dePriester JA, Cole TJ, Lucas A. HW #5, WB.1 - Pxy Diagram and Henry's Law Constant. For daily clinical use, an individual may be plotted on centile charts like those shown in figs 1 and 2.HW #5, P12.3 - Fitting VLE Data Using the Margules.But I only got to something like 4e-4 in one try.
DEPRIESTER CHART ONLINE PDF
docx), PDF File (.pdf), Text File (.txt) or read online for free.
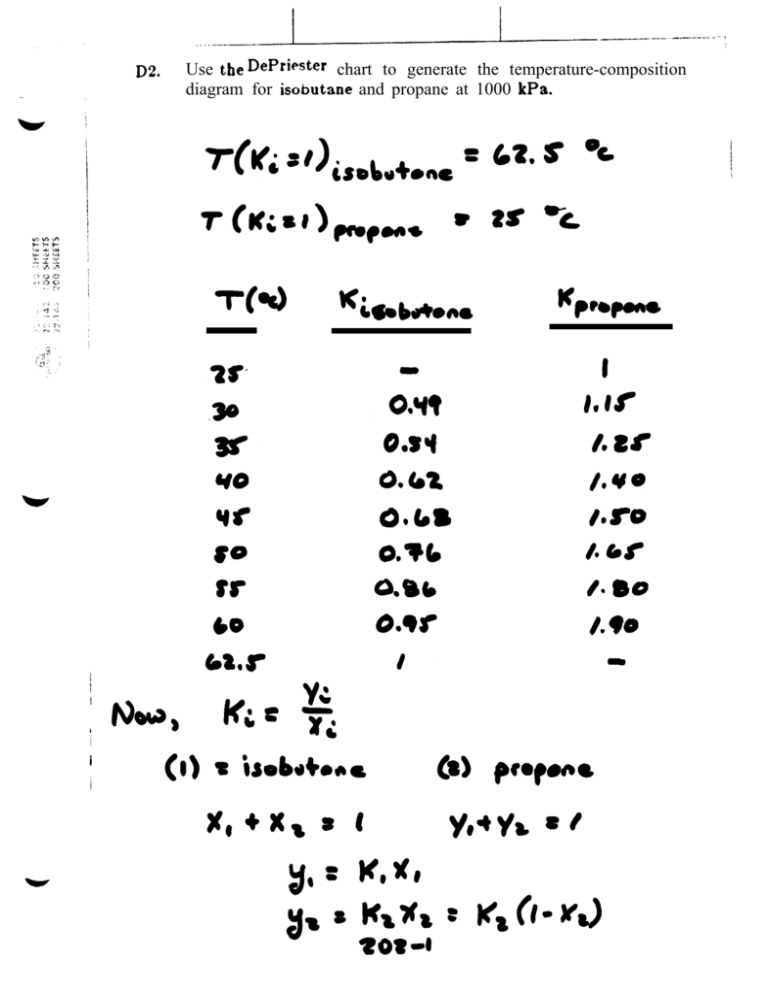
You can also run solver on each eqn, one at a time and then go back and do all 5 together. P and P calculation based on the DePriester chart for a. I you want, you can go onto the options tab and change the Estimates, Derivatives and Search methods. That should get you very close to the right answer in one pass in Solver. Constrain both Zs to be > 0.04 AND constrain BOTH f'(Z) functions to be > 0 (to make sure the two roots you find are the sat vap and sat liq roots and not the meaningless one). The easiest way to get Solver to go where you want it is to add FOUR constraints. This is something like the %RMS error for the 5 eqns. I minimized SQRT of the sum of these 5 error terms (divided by 5. Right ? I used %error^2 for each equilibrium eqn and f(Z)^2 for the SRK eqns. You should also have 5 unks: Zvap, Zliq, P, x1 and x2. any suggestions." I replied: OK, you have 5 eqns: 3 of the them are yliq The remaining 2 eqns are SRK eqns: one for liquid and one for vapor. I set a constraint for solver that Z is greater than B, but it won't listen. The concentration of i- C 5 should be <1 mole of total C 4 ’s and lighter compounds in the distillate product.
It represents the steady-state heat flow through a unit area of. Part 1: Using the problem definition given in Figure 5.3 below, calculate the n-C 4 flow rate in the distillate product, if the debutanizer column has 18 (actual) plates. Take the equiIibrium data from thé Depriester charts givén in Chapter 8. Calculate the fIow and composition óf the liquid ánd vapour phases. The DePriester chárts 80 check this quite well (see Figures 8-4A and B, and Figure 8-3D). It is a fundamental property, independent of the quantity of material. For n-péntane at convergence préssure of 3,000 psia (nearest chart) the K-value reads 0.19. A k-value (sometimes referred to as a k-factor or lambda value ) is a measure of the thermal conductivity of a material, that is, how easily heat passes across it. total pressure K 1.0 temperature DePriester Chart for a given P, find Tbp (i.e., K 1) for a given T, find Psat (i.e., K 1) for a given P, T, find K K > 1 prefers vapor phase K < 1 prefers liquid phase Don’t extrapolate beyond the range of the chart. I get num! I think this is because Zliq is below Bliq and the same for vapor. For an alternative meaning, see kappa value. A student emailed me the following question: "I have all my 5 equations set up and am trying to simultaneously solve for the unknowns.


 0 kommentar(er)
0 kommentar(er)
